Draw The Structure Of Co32
What is Resonance Structure
We can draw two or more Lewis structures for some molecules and polyatomic ions, without changing the position of atoms in the structure. In this case, only the electron distribution differs from one structure to another. These structures are called resonance structures or contributing structures. But, the actual molecule has an intermediate structure of those possible Lewis structures.
The first step of drawing resonance structures starts with drawing all the possible Lewis structures. If it has only one Lewis structure, it doesn't have a resonance hybrid.
How to Identify the Molecules Having Resonance
Resonance exists only when a Lewis structure has multiple bonds and an adjacent atom with at least one lone pair. The general form of resonance is illustrated below. Arrows are used to indicate the shifting of electrons from one resonance structure to another.

How to Draw Resonance Structures
Lewis Structures
Example 1: CO3 2-ion
Step 1
Calculate the total number of valence electrons from each atom.
Carbon atom = 4
Oxygen atoms (3*6) = 18
For (-2) charge = 2
** Consider the -2 charge at the last step (i.e. this molecule has two extra electrons).
Step 2
When there is more than one type of atom, keep the least electronegative or metallic atom as the central atom.
Carbon is the central atom in CO3 2- ion
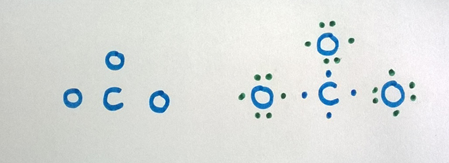
Step 3
Combine each atom with a single bond to the central atom by contributing one electron from each atom for the bond.
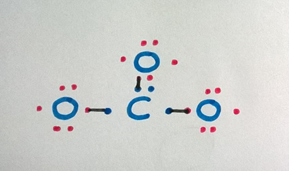
Step 4
Count the electrons in the valence shell to check if the octet is completed.
The carbon atom needs one more electron, and each oxygen atom needs one more electron to complete the octet.
Step 5
If not, add some more bonds until all octets are full.
** Leave the non-bonded electrons as dots and draw a single line (-) for a single bond and two lines (=) for a double bond.
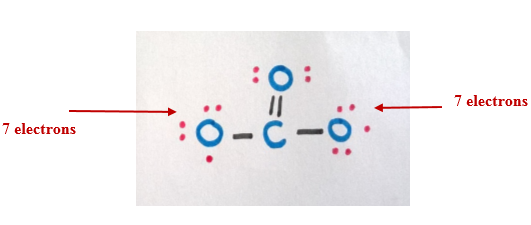
If we add one bond between the carbon atom and an oxygen atom, both carbon atom and the oxygen atom complete the octet.
Step 6
Now consider the -2 charge (two additional electrons). Since the other oxygen atoms have only 7-electrons in their outermost shell, we can distribute those two electrons among them.
The final structure can be written as follows.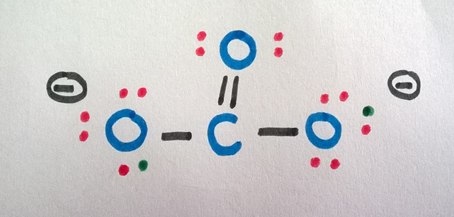
Step 7
Now, we can draw the possible resonance structures as discussed in section 1.
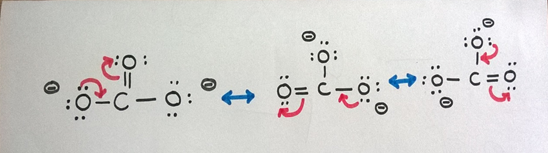
We can draw three resonance structures for CO3 2- ion as above.
Let's take two more examples to learn how to draw resonance structures.
Example 2: O3 Molecule
We can use the same procedure as outlined above to obtain the Lewis structure. It gives the following structure, and has a multiple bonds and an adjacent atom with one lone pair of electrons.

Therefore, we can draw resonance structures for O3 molecule as follows.
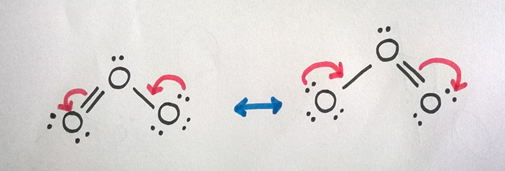
Example 3: Carboxylic Acid
We can obtain the following resonance structures, by following the same steps as mentioned above.
Definitions:
Lewis structure: A simple method of representing the configuration of atoms in a molecule, showing lone pairs of electrons and the bonds between atoms.
References:
Writing Resonance Structures. (n.d.). Retrieved November 15, 2016, from here
Ch 1 : Resonance. (n.d.). Retrieved November 15, 2016, from here
A Brief Tutorial on Drawing Lewis Dot Structures. (n.d). Retrieved November 15, 2016, from here
Bishop, M. (n.d.). Resonance. Retrieved November 15, 2016, from here

You May Also Like These
Draw The Structure Of Co32
Source: https://pediaa.com/how-to-draw-resonance-structures/
Posted by: sheltonhemperess.blogspot.com

0 Response to "Draw The Structure Of Co32"
Post a Comment